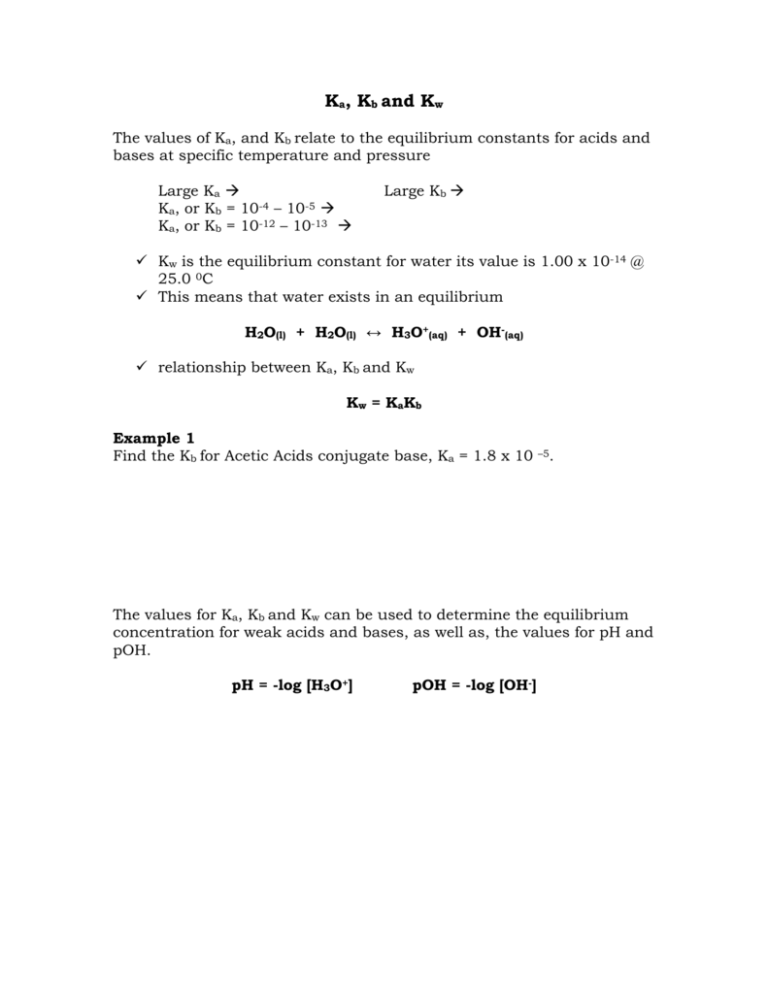
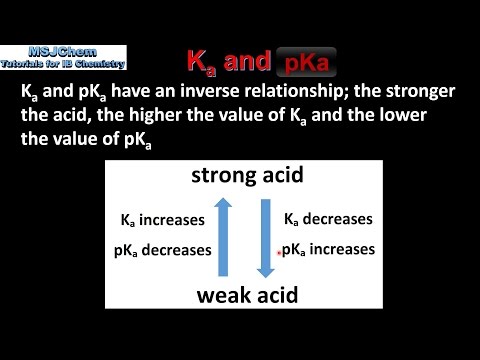
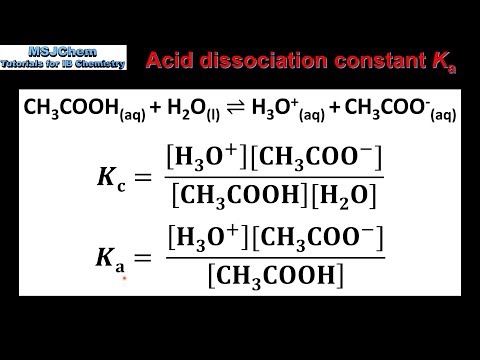
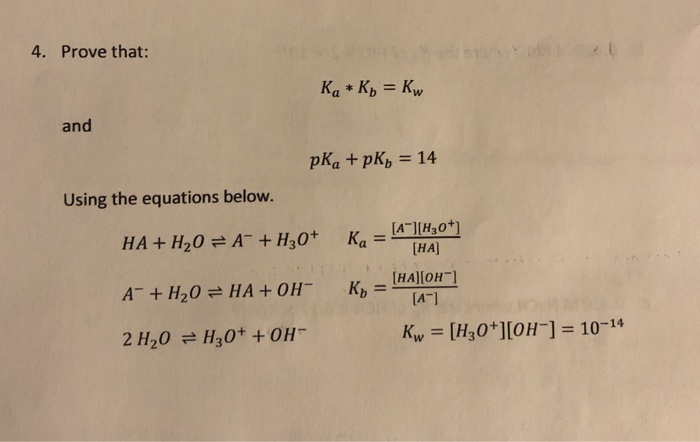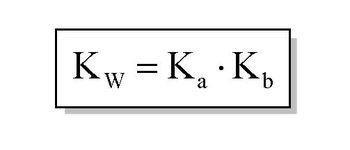CALCULATING Ka Kb Kw, ICE Tables, ACID DISSOCIATION CONSTANT Power Points & M.C. WITH ANSWERS (59PGS) | Teaching Resources

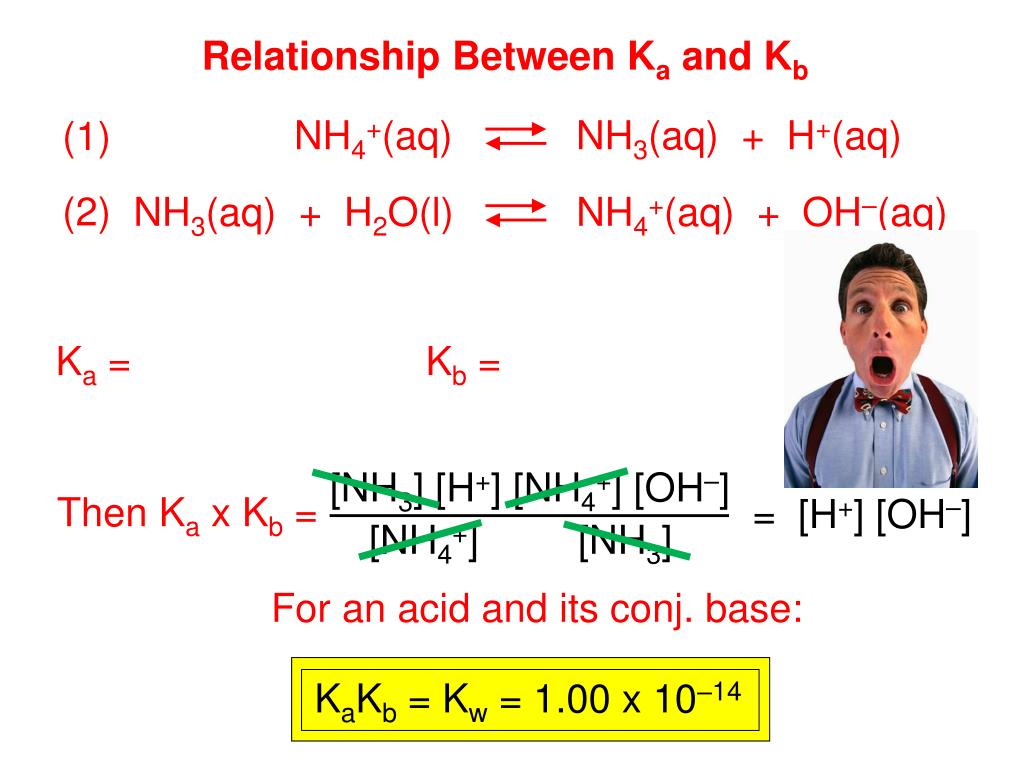
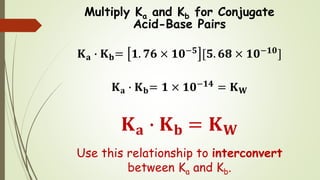
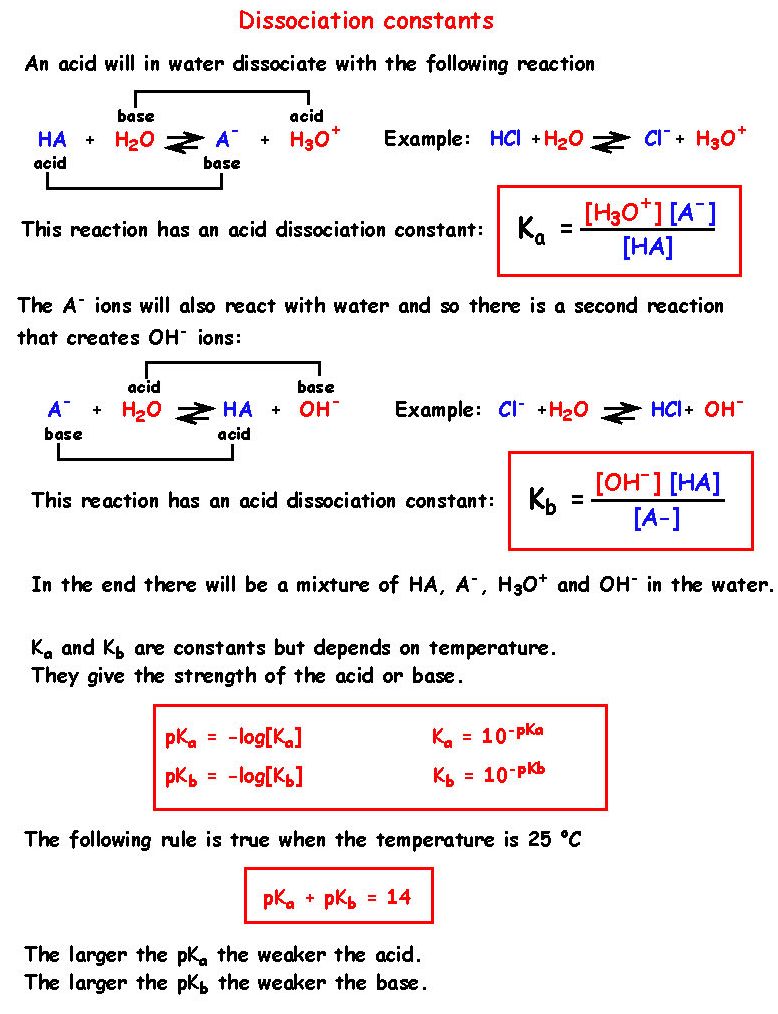
From the dissociation constants Ka and Kb for an acid and its conjugate base, show that Ka· Kb = Kw .
![SOLVED: Helpful Equations pH = -log [H2O+] Kw = Ka × Kb [ATJ[H2O+1 [HA]] = pOH -log [OH-] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson-Hasselbalch Equation [A-] pH = pKa log - [HA] SOLVED: Helpful Equations pH = -log [H2O+] Kw = Ka × Kb [ATJ[H2O+1 [HA]] = pOH -log [OH-] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson-Hasselbalch Equation [A-] pH = pKa log - [HA]](https://cdn.numerade.com/ask_images/9f4de4f19628424f8aea53cff3ecadcb.jpg)
SOLVED: Helpful Equations pH = -log [H2O+] Kw = Ka × Kb [ATJ[H2O+1 [HA]] = pOH -log [OH-] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson-Hasselbalch Equation [A-] pH = pKa log - [HA]




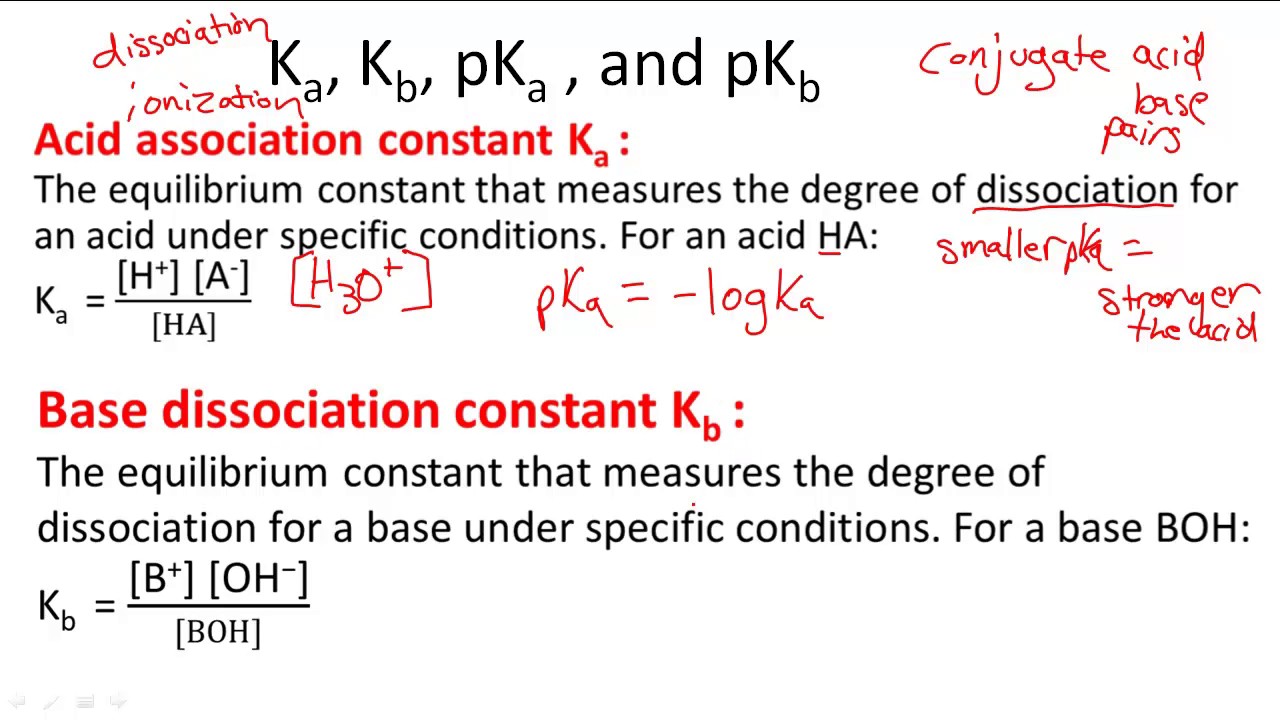
![K[a], K[b], pK[a] , and pK[b] - Overview ( Video ) | Chemistry | CK-12 Foundation K[a], K[b], pK[a] , and pK[b] - Overview ( Video ) | Chemistry | CK-12 Foundation](https://s3.amazonaws.com/ck12bg.ck12.org/curriculum/107681/thumb_540_50.jpg)